Research could enable improved treatment of spinal cord injuries
October 23, 2019
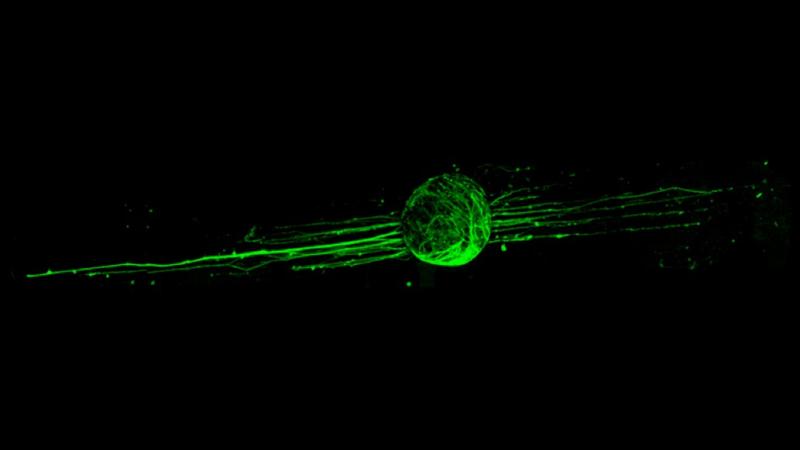
TROY, N.Y. — Spinal cord damage that causes paralysis and reduced mobility doesn’t always stop with the initial trauma, but there are few treatment options to halt increased deterioration — and there is no cure. Researchers at Rensselaer Polytechnic Institute have developed a promising new biomaterial that could offer targeted treatment to the damaged spinal cord and tissue, preventing further damage.
In research published today in Nature Communications, an interdisciplinary team from Rensselaer demonstrated how estrogen — a natural hormone produced in the body — can be polymerized into a slow-releasing biomaterial and applied to nervous system cells to protect those cells and even promote regeneration.
“Estrogen is known to be neuroprotective,” said Ryan Gilbert, a professor of biomedical engineering at Rensselaer and a member of the Center for Biotechnology and Interdisciplinary Studies (CBIS). “After spinal cord injury, you have all these free radicals that are released and cause the injury to increase over time. We’re trying to stop the spread of the injury. It’s more of an acute phase treatment we are looking to develop.”
By observing nature’s methods of protection, Gilbert partnered with Edmund Palermo, an assistant professor of materials science and engineering at Rensselaer, to develop a polymer that — when implanted directly on the spinal cord — would target the injured tissue and release estrogen as a therapeutic over a period of years.
The approach is more precise than traditional drugs that often impact the entire system and may induce side effects in other organs.
“Using natural materials in ways that release it, or introduce it, to tissue in interesting ways can really have a lot of benefit, and that’s what we’re seeing in our work here,” Gilbert said.
Conventional methods for making drugs into polymers, known as polypro drugs, weren’t ideal for this application, so Palermo and his team developed a new approach. They used a photo-triggered chemical method to synthesize the estrogen into long polymer chains that — using electric force — could be spun into fibers necessary for implantation along the spinal cord.
In order to test these newly synthesized fibers, Gilbert and his team applied nervous system cells like neurons and astrocytes to the polymerized estrogen. Through this cellular lab testing, the researchers found that estrogen wasn’t only neuroprotective, but also might promote regeneration.
“This was the first time polyestrogen was processed into fibers that showed the ability to enhance the outgrowth of neural cells along the fiber direction without adding growth factors,” Palermo said.
The team’s new approach is now being patented and will enable the researchers to push their exploration even further toward preclinical research, where they can see how their polymerized fibers would work in a living system. Their eventual goal is to vastly improve the lives of people with spinal cord injuries.
Their findings will also help advance research in the area of drug delivery, which is increasingly focused on personalization and precision.
“Precision medicine is a research priority within CBIS,” said Deepak Vashishth, director of CBIS, a research center that brings faculty from multiple disciplines together to solve challenging global problems. “What our researchers have developed here has the potential to advance a previously intractable challenge.”
“The type of synthetic chemistry that we developed is broadly applicable to the slow release of many other drugs,” Palermo said.
“Drug delivery to the injured spinal cord is a really challenging issue,” Gilbert said. “I think the next stage of drug delivery will be very focused treatments that can be applied directly to the injured spinal cord so that side effects are greatly reduced.”
Devan Puhl and Anthony D’Amato, graduate students in the department of biomedical engineering and in CBIS, and Samuel Ellman, a graduate student in the department of materials science and engineering, all contributed to this research.
The work was supported, in part, by a grant from the National Institute of Neurological Disorders and Stroke, NSF CAREER grants, and a grant from the New York State Spinal Cord Injury Research Board.