Goal is to produce a low-dose, oral medication that can be taken at home
June 27, 2022
Rensselaer Polytechnic Institute researchers Gaetano Montelione and Christopher Cioffi will use a five-year, $3.5 million grant from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), to develop a low-dose, oral COVID antiviral drug that can be administered at home. Dr. Montelione is the Constellation Endowed Chair of Structural Bioinformatics and Dr. Cioffi is the Thomas and Constance D’Ambra Endowed Chair of Organic Chemistry. Both are professors in the Department of Chemistry and Chemical Biology.
Rensselaer’s research is part of the consortium of a new antiviral drug development center, called the Center for Antiviral Medicines and Pandemic Preparedness (CAMPP), that will be led by Scripps Research. The center will be one of nine NIAID-sponsored Antiviral Drug Discovery (AViDD) Centers for Pathogens of Pandemic Concern.
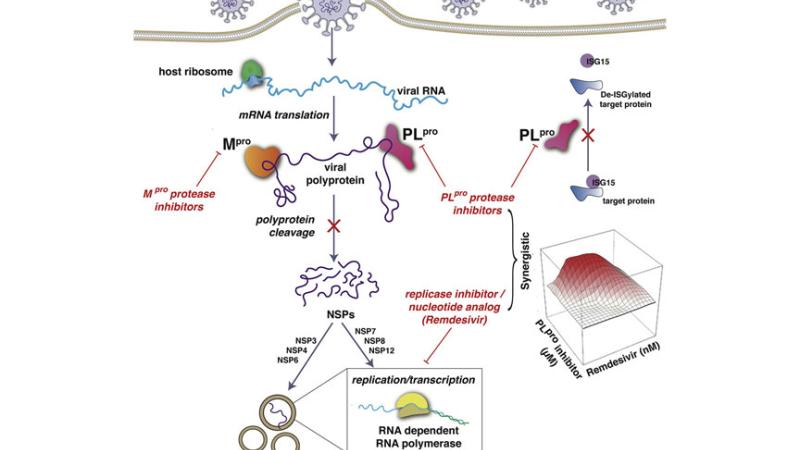
“Our role in the project is to develop novel inhibitors of the two key proteases of the SARS-CoV-2 virus, called CLpro and PLpro, which are essential to the virus life cycle,” Dr. Montelione said.
The Rensselaer team will collaborate with Drs. Sumit Chanda and Arnab Chatterjee of Scripps Research Institute, Dr. Adolfo Garcia-Sastre of the Mount Sinai School of Medicine, and Dr. Stefano Ciurli of the University of Bologna, Italy.
The team’s work will build upon Dr. Montelione’s previous research. First, using bioinformatics, Dr. Montelione and his team found that a key protein from the hepatitis C virus closely resembles the coronavirus CLpro protease structure. Since several Food and Drug Administration (FDA)-approved drugs target the hepatitis C protease, the next step was to determine if any of those drugs would also bind and block proteases of SARS-CoV-2.
In research published in Cell Reports, the team found that, out of the 10 hepatitis C drugs tested, seven suppressed the SARS-CoV-2 virus. Three of those drugs were acting not only on the main protease, CLpro, but on the PLpro protease, as well. Next, they discovered that, when combined with the polymerase inhibitor remdesivir, these drugs multiplied remdesivir’s antiviral activity by as much as tenfold. Those that only inhibit CLpro did not amplify remdesivir’s effect.
“Inhibiting PLpro seems to be important,” Dr. Cioffi said. “This induces synergy with other antivirals such as remdesivir, which is currently being used to treat people with COVID-19. Now, we will work toward making new and orally bioavailable PLpro inhibitors based on Dr. Montelione’s biophysical analysis. We will look at the structure of the protein’s active site and design molecules that fit into it and bind with optimal affinity.”
CLpro will also be targeted.
“One of the big challenges is that the target will evolve to escape the drug,” Dr. Montelione said. “This is particularly problematic with rapidly mutating viruses like COVID-19. To overcome that, we try to hit multiple targets. By hitting both CLpro and PLpro simultaneously, there is less likelihood of antiviral resistance.”
“Rensselaer is fortunate to have two distinguished faculty members working together on complementary aspects of a new COVID-19 therapy,” said Dr. Curt Breneman, Dean of the School of Science. “Through their innovative strategy of designing tandem protease inhibitors, new and highly effective COVID-19 treatments are now on the horizon.”
It is hoped that the most promising antiviral drug candidates to come out of the AViDD centers will enter late-stage preclinical development. Industry partners will provide valuable resources to accelerate their movement through the product development pipeline. Drugs that are already FDA-approved for treating different diseases may be approved quicker for the treatment of COVID-19, as well.
The Rensselaer research team will include Thomas Acton, Khushboo Bafna, Namita Dube, Rebecca Green-Cramer, Arunan Palanimuthu, and Theresa Ramelot.