New study sheds light on how Reelin signaling can protect against Alzheimer’s and more
February 9, 2026

A team of researchers at RPI, in collaboration with the University of South Florida, University of North Carolina, and The Neural Stem Cell Institute, have made a discovery that opens the door to new treatments for Alzheimer’s disease and other brain disorders.
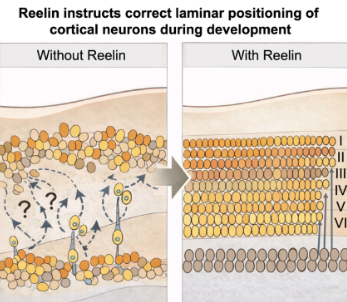
The research, published in the Journal of the American Chemical Society, reveals a critical mechanism in how a brain signaling pathway communicates at the molecular level, and suggests that enhancing that communication could protect the brain from damage related to neurological diseases. The study focuses on the protein Reelin, which helps neurons stay organized during brain development and supports learning and memory in adulthood (see diagram).
The RPI-led research team found that in order for Reelin to function properly in the brain, it must interact with a special cell surface sugar molecule called heparan sulfate (HS) — specifically, a version of HS that has a chemical feature called N-sulfation.
“Without N-sulfation, Reelin cannot send its messages effectively, and the brain’s protective signaling is compromised,” said Lin Pan, Ph.D., lead author on the study and postdoctoral researcher at RPI.
This research builds on an earlier discovery involving a protective Reelin variant known as COLBOS. The COLBOS variant was first identified in 2023 in a patient carrying a genetic mutation that should have caused early-onset Alzheimer’s but didn’t, allowing the patient to remain dementia-free for decades longer than expected. At the time, it was unclear why the patient was protected, but researchers now think one possible reason may be the COLBOS variant’s stronger interaction with HS.
In this new study, the researchers found that full-length COLBOS Reelin protein binds even more strongly to the N-sulfated HS on cells. “This interaction likely enhances protective Reelin signaling, which may in turn help slow the progression of Alzheimer’s disease-related pathology, including elevated tau phosphorylation and the formation of neurofibrillary tangles,” said Pan.
“This is the first study to reveal the significance of specific HS sulfation patterns in Reelin-HS interaction, said Chunyu Wang, Ph.D., corresponding author on this study and professor of biological sciences at RPI. “We are confident that these findings will help advance Reelin-related Alzheimer’s disease research and provide a deeper understanding of the role of protein–HS interactions.”
This new understanding about Reelin, how it binds to HS, and the impacts of enhancing Reelin-HS interaction opens the door to potential new treatments for a range of neurological disorders including Alzheimer’s, schizophrenia, epilepsy, and more. Knowing that stronger interactions between Reelin and HS can help protect the brain, future therapies could focus on increasing HS N-sulfation or designing drugs that mimic the COLBOS variant to strengthen Reelin signaling.