August 10, 2022
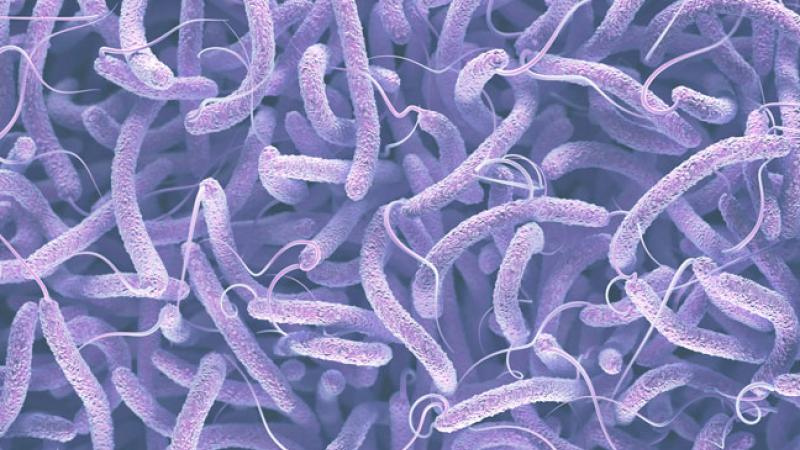
Cholera is a life-threatening disease caused by the bacterium Vibrio cholerae that appears seasonally in some regions of the world and during natural or human-made disasters. The emergence of drug-resistant bacteria against which no antibacterial drugs are effective has become a global problem. Thus, searching for new drug targets and candidate compounds is a high-priority task.
Rensselaer Polytechnic Institute’s Blanca Barquera, professor in the department of biological sciences, and Nicole Butler, graduate student in the department of chemistry, collaborated with Hideto Miyoshi of Kyoto University and Junchi Kishikawa of Osaka University to obtain a detailed structure of the respiratory enzyme Na+-pumping NADH-quinone oxidoreductase (NQR) from Vibrio cholerae. Their findings were recently published in Nature Communications. NQR is essential for the energy production of some pathogenic bacteria, including Vibrio cholerae.
“The detailed structure allows us to clarify how the enzyme works in harnessing energy for the cell,” Barquera said.
The team used cryo-electron microscopy methods with a 2-3A resolution. Inhibitors of the enzyme were used to stabilize the structure, allowing previously unresolved parts of the enzyme to be visualized. The structural details of inhibitor binding are essential to understanding the interaction of antibacterial drug candidates with the enzyme.