Findings yield a new target in the fight against drug-resistant cancer
July 16, 2025
A discovery with major implications for cancer treatment has been made by a team of researchers from Rensselaer Polytechnic Institute (RPI), the University of Nebraska Medical Center (UNMC), University of Binghamton, and SUNY College of Environmental Science and Forestry. These researchers are focused on a biological pathway called Hedgehog signaling, which has nothing to do with small animals and everything to do with how cells grow and divide.
The key to improving cancer treatment may lie in developing new drugs that more effectively block this process of Hedgehog signaling — beginning with the generation of the Hedgehog ligand, the protein that triggers the entire pathway. This biological pathway, which helps guide cell growth during early development, can become dangerously overactive in adults, driving the malignant growth of various types of common cancers, including prostate, lung, pancreatic, and ovarian. While drugs that inhibit Hedgehog signaling already exist, cancer cells often develop resistance to them — making it essential to better understand how the pathway functions and to identify new treatment targets and strategies.
“Our team is uncovering key details in the Hedgehog pathway that bring us closer to new, more effective cancer treatments,” said Chunyu Wang, Ph.D., professor of biological sciences at RPI. “This deeper understanding provides fresh opportunities to tackle cancers that have been difficult to treat.”
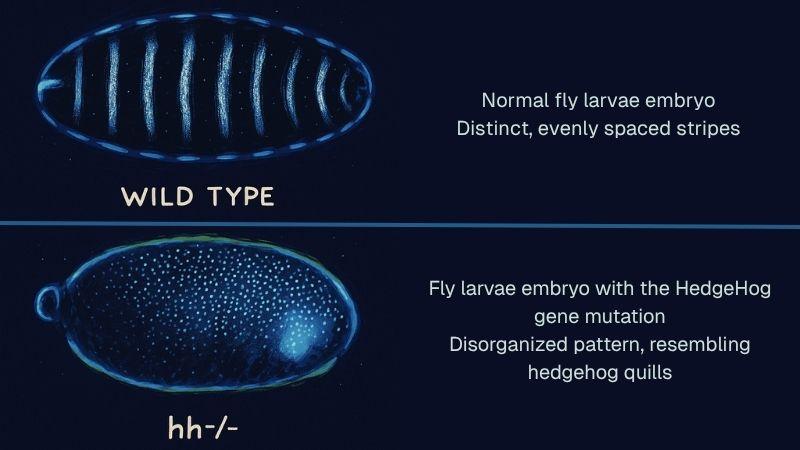
The Hedgehog signaling pathway is a key biological system that directs how cells grow, divide, and take on specialized roles during early development. It was first discovered in fruit flies in 1980 by scientists studying how genes control body formation. They observed that fly larvae with mutations in a particular gene developed short, spiky projections on their bodies — resembling the quills of a hedgehog — and named the gene accordingly. Later research showed that vertebrates have several related Hedgehog genes, all of which play essential roles in shaping the body during embryonic development. When this signaling process is disrupted, it can lead to severe birth defects — such as extra limbs or even a single eye — and is now known to contribute to the pathogenesis of many types of cancer when abnormally reactivated in adults.
The critical role of this pathway was famously illustrated in the 1950s, when sheep grazing on certain plants in Idaho gave birth to lambs with a single eye — a condition known as cyclopia. Scientists later discovered that a compound in the plants (appropriately named cyclopamine) blocked Hedgehog signaling, disrupting normal brain and facial development. This example highlights how essential the pathway is in early development — and why it must be tightly regulated throughout life.
In a study published in the Proceedings of the National Academy of Sciences, the research team uncovered a previously hidden step in the way the Hedgehog ligand is generated — a biochemical process known as autoprocessing, which is required for the ligand to function and initiate signaling. At the center of this process is a specific site on the Hedgehog protein — an amino acid called cysteine 143 (C143) — which forms a temporary, branched chemical structure essential for the protein’s function.
Using advanced methods including mass spectrometry (MS) and nuclear magnetic resonance (NMR), the researchers demonstrated that this branched intermediate is formed as a thioester bond on the side chain of C143. The unique chemical reactivity of C143 makes it a strong candidate for covalent inhibitors — drugs that bind tightly and irreversibly to their targets and block activity permanently.
“Advanced technologies like high-resolution LC-MS/MS and high-field NMR form an ideal complementary and synergy toolbox, and this study is a clear example,” said Ke Xia, Ph.D., director of the Proteomics Core at RPI’s Center for Biotechnology and Interdisciplinary Sciences (CBIS). “Using high-resolution LC-MS/MS, we captured a fleeting but critical intermediate in the Hedgehog activation” — an insight made possible by the CBIS core’s Orbitrap Eclipse Tribrid and Orbitrap XL spectrometers.
By understanding this newly revealed mechanism, scientists may now be able to design drugs that disable Hedgehog signaling at its source — offering new hope for treating cancers that rely on this pathway, especially those that have stopped responding to existing therapies.
The research team was led by RPI professor Chunyu Wang, Ph.D., M.D. and working alongside him as principal investigator (PI) was Wang’s past student and Ph.D. mentee, Jian Xie ’15 Ph.D., assistant professor at UNMC.
“Cancer remains one of the leading causes of death worldwide and can be driven by environmental exposures, genetic alterations, and viral infections. The Hedgehog signaling pathway is implicated in all these aspects of carcinogenesis and disease progression. Building on a series of important discoveries and a robust, orthogonal technical pipeline we have established, this latest study marks a critical milestone toward the development of a novel cancer therapeutic strategy,” said Xie. “This work also represents a culmination of years of collaborative research and technical innovation.”
Shannon Faris ’24, Ph.D., (Chemistry) and Nathan Smith ’25, Ph.D. (Biochemistry and Biophysics) contributed to the research as they were completing their doctoral studies at RPI.
"This paper represents the culmination of years of persistence and problem-solving during my Ph.D.,” said Faris, who graduated in summer 2024. “What’s most exciting is that this discovery opens up a new strategy for targeting cancer — one that could have real therapeutic impact for patients."
“Getting the opportunity to play such a meaningful role in the research we published in PNAS, especially right as I was finishing my Ph.D., is both humbling and exhilarating,” said Smith. “It reminds me that even early career scientists can push the bounds of what we know.”
To further inspire young people to explore opportunities in STEM, Wang invited area high school students into his lab, helping them gain early exposure to impactful scientific research. The research team also included Brian Callahan, Ph.D., associate professor at the University of Binghamton, and José-Luis Giner, Ph.D., associate professor at the SUNY College of Environmental Science and Forestry.
This research, funded by the National Institute of Health, provides a clearer understanding of Hedgehog ligand generation and activation — and a new target in the fight against drug-resistant cancer.
Read the full paper here: https://www.pnas.org/doi/10.1073/pnas.2415144122
